25 years ago, on March 27, 1998, the U.S. Food and Drug Administration (FDA) approved Viagra for treatment of male erectile dysfunction. With its distinct blue diamond shape, Viagra quickly gained notoriety and became deeply ingrained in popular culture. For Pfizer, the drug was an instant success, surpassing $1 billion in global sales in its second year on the market and remaining one of the company’s best-selling drugs for years to come.
Originally studied for use in hypertension (high blood pressure) and chest pain associated with coronary heart disease, sildenafil, which is the generic name of the drug later marketed as Viagra, was found to sometimes induce penile erections during clinical trials. Seeing an opportunity, Pfizer decided to study and market it for erectile dysfunction, in a move that became a textbook example of drug repositioning.
While Viagra is still one of the most recognizable drugs in the world and synonymous with sexual performance enhancement, its success story began to fade in 2013 when Pfizer’s patent on the use of sildenafil in erectile dysfunction expired in the European Union. Around the same time, the company was involved in a patent lawsuit in the United States, which resulted in a settlement allowing Teva Pharmaceuticals to launch a generic version of Viagra in December 2017.
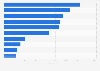
As our chart illustrates, the loss of exclusivity in major markets such as Europe, Japan and most importantly the United States had a significant effect on Viagra sales, which declined by almost 70 percent between 2012 and 2018. 25 years after its initial approval, Viagra is no longer part of Pfizer either. In 2020, the company’s off-patent branded and generics business Upjohn, which included Viagra, was spun-off and combined with Mylan to create a new company called Viatris.