While governments around the world are taking measures to slow down the COVID-19 pandemic, scientists are working vigorously to find a long-term solution, meaning either a drug that helps alleviate the illness or a vaccine.
While the world’s hopes for an effective treatment for COVID-19 are currently resting on an antiviral drug called remdesivir, which was originally developed to fight Ebola and is currently tested against COVID-19, experts have pointed out that a vaccine won’t arrive soon enough to help with the current outbreak.
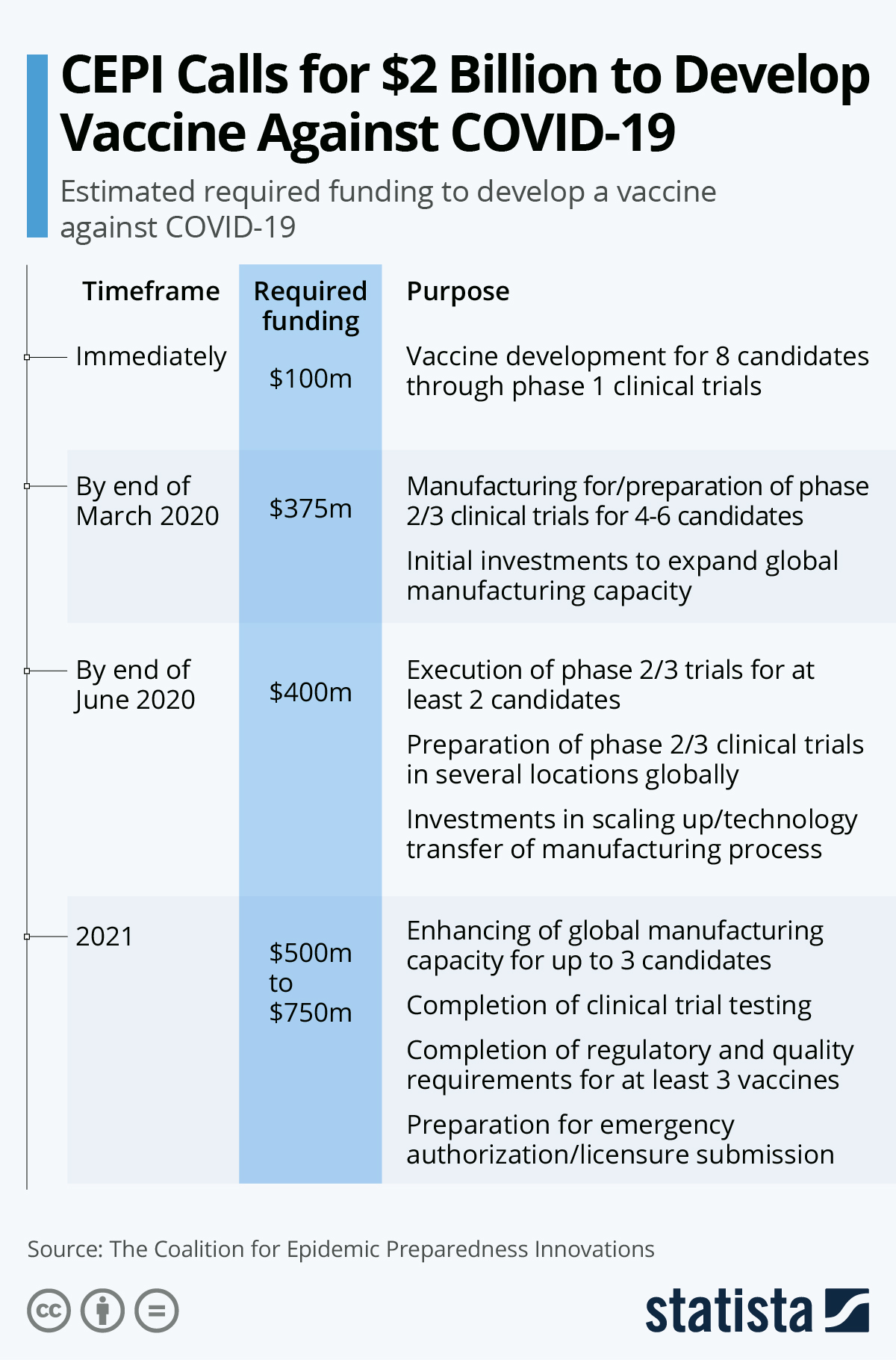
On Saturday, the Coalition for Epidemic Preparedness Innovations (CEPI), an organization set up to accelerate the development of vaccines against emerging infectious diseases, has laid out a possible roadmap for the development of a vaccine, calling for $2 billion in funding, required in different phases, starting with an initial investment of $100 million to kick off development of 8 candidates.
“The rapid global spread and unique epidemiological characteristics of the novel coronavirus is deeply concerning,” CEPI writes on its website. ”So far, we have initiated 6 partnerships to improve our understanding and to develop vaccines against the novel coronavirus. The aim is to advance COVID-19 vaccine candidates into clinical testing as quickly as possible.”
As the following chart shows, a vaccine likely won't be ready this year, however. The last stage laid out in the CEPI's roadmap includes the conclusion of clinical trials, the enhancement of manufacturing capacities and the preparation for emergency authorization/licensure submission - unfortunately that stage is scheduled for 2021.